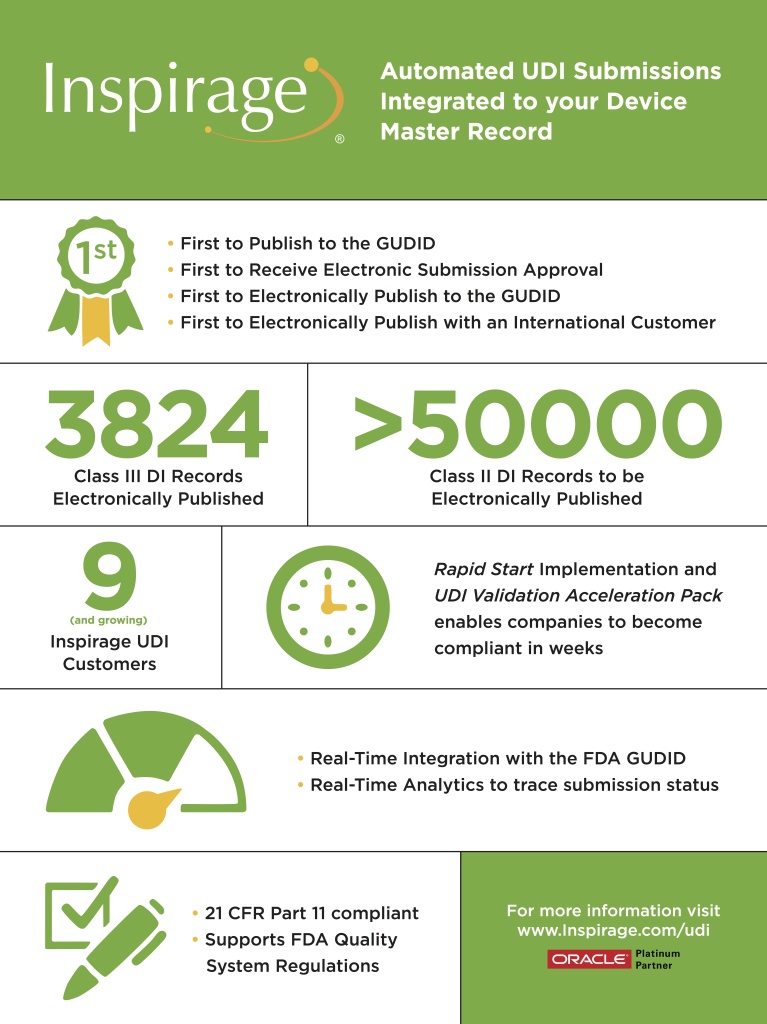
Inspirage is the leader in helping medical device companies become UDI compliant. Based on extensive collaboration with industry experts and leading medical device companies, Inspirage developed a comprehensive solution leveraging Oracle Agile PLM that combines enhanced business process enablement with data capture to achieve UDI compliance. This solution delivers a rapid, cost-effective approach to manage the capture and control of the (D.I.) attributes to solve FDA registration requirements, as well as synchronizing (P.I.) attributes to assure internal traceability across systems. Learn more >>
Mike Lieberman | Key Contributor
Mike Lieberman is a Director of Solution Architecture for Inspirage. Mike is focused on architecting robust value chain solutions, focusing on Agile PLM; creating and positioning innovative solution offerings to enable a customer to expand their PLM footprint; delivering deep product expertise and value to the Inspirage client base; and focusing on the sales and business development activities for PLM consulting opportunities.